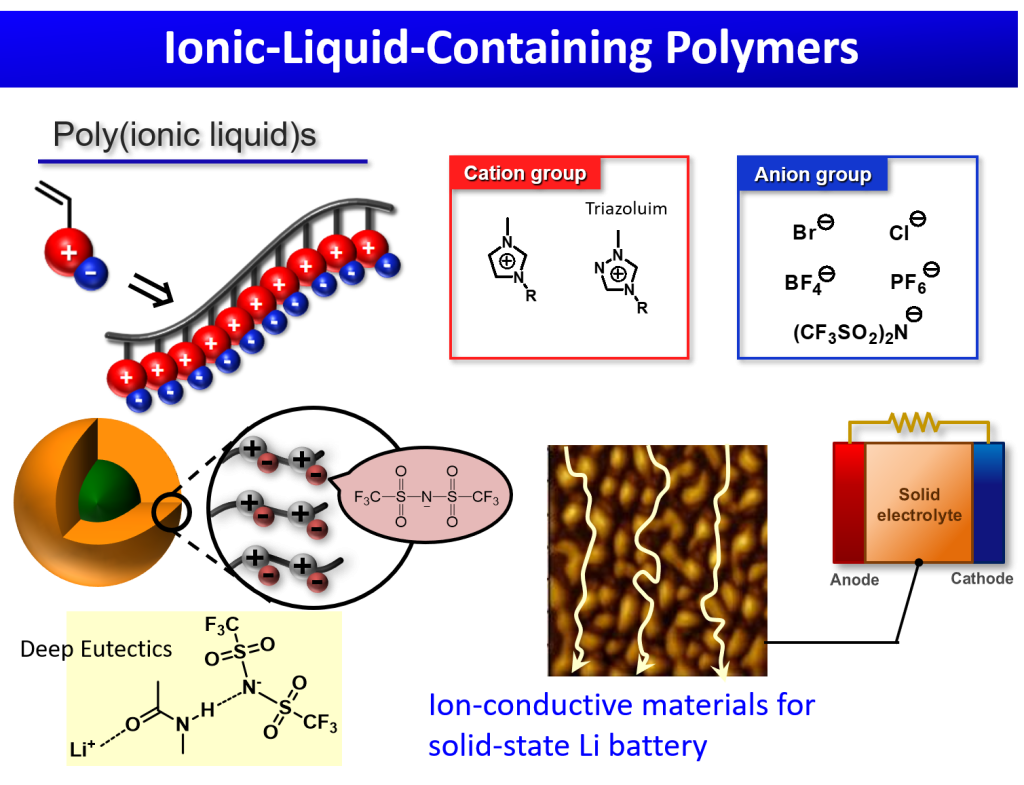
Ionic liquids are organic salts with low melting points that have many characteristic properties including high ionic conductivity, high polarity, high density, large heat capacity, and excellent thermal and chemical stabilities. We are interested in the synthesis and practical applications of polymeric ionic liquids with unique and attractive properties that can be easily tuned by modifying the cation (e.g., imidazolium, triazolium, and tetraalkylammonium) and anion (e.g., halide, tetrafluoroborate, hexafluorophosphate) structures. We have developed imidazolium ionic-liquid-based block and star-block copolymers that show a characteristic thermally induced phase separation behavior and form self-assembled structures in an aqueous solution. In addition, by changing the counter anion (from hydrophilic Br- ion to hydrophobic bis(trifluoromethanesulfonyl)imide (Tf2N-) ion) via an ion exchange reaction, we successfully developed imidazolium ionic-liquid-based block copolymers as promising ionic conductors. The unique ionic conductivity and various other attractive properties of 1,2,4-triazolium-containing ionic liquids enabled us to develop ion conductive block copolymers and core-cross-linked core-shell nanoparticles via the RAFT polymerization of N-vinyl-1,2,4-triazolium salts. Our recent interests involve the integration of polymer chemistry and deep eutectic solvents (DESs), which can be considered as a subclass of ionic liquids. DES are generally produced by mixing an organic salt (which acts as a hydrogen bond acceptor) with a hydrogen bond donor.